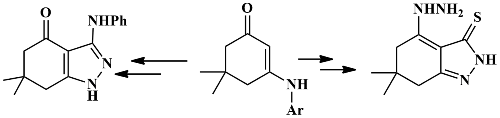
Synthesis of tetrahydroindazol-4(5H)one and 7-thione from reaction of functionalized cyclic enaminones with hydrazine
Abstract
Full Text:
PDFReferences
- E. S. H El Ashry, L.F. Awad, Y. El kilany, E. I. Ibrahim, Dimedone: A Versatile Precursor for Annulated Heterocycles, Adv. Heterocycl. Chem., 2009, 98, 3- 141.
- M. S. Bashandy, Synthesis, Anti-Breast Cancer and Molecular Docking of Some Heterocycles Incorporating N, N-Dibenzylbdenzensulfonamide, Int. J. Adv. Res., 2014, 2, 436-469
- E. A. Ragab, N. H. Metwally, M. S. Mohamed, Synthesis of Some Novel Pyrazolo[1,5-[a]quinazolines and Their Fused Derivatives, Synth. Commun., 2017, 47,148-158.
- S.S. Asma; B. Kalluraya, Enaminones as Building Block: Synthesis of Novel Substituted Pyrazoles as Possible Antioxidants, Indian J. chem., 2016, 55B, 501.
- Y. Zhao, Q. Duan, Y. Zhou, Q. Yao, Z. Li, Gold-Catalyzed Chemo- and Diastereoselective C (sp2 )- Functionalization of Enaminones for the Synthesis of Pyrrolo[3,4-c]-quinolin-1-one Derivatives, Org. Biomol. Chem., 2016, 14, 2177-2181.
- A.A. Hassan, Heterocyclic Synthesis Via Enaminones: Synthesis and Molecular Docking Studies of Some Novel Heterocyclic Compounds Containing Sulfonamide Moiety, Inter. J. Org. Chem., 2014, 4, 68-81.
- L. Long, Y. Shao, Y. Li, Y. Liu, Y. Li, LDA-Promoted Synthesis of 3-Amino Furans by Selective Lithiation of Enaminones, J. Org. Chem., 2015, 80, 12641-
- L.V. Sokolenko, Y. L. Yagupolskii, L. S.Kumanetska, J. Marrot, E. Magnier, V. O. Lipetskij, I. V. Kalinin, CF3 (O)n- Containing enaminones as Precursors for The Synthesis of Pyrimidine-4(3H)-ones, Tetrahedron Lett., 2017, 58, 1308- 1311.
- Y, Tominaga, Synthesis of Heterocyclic Compounds Using Carbon Disulfide and Their Products, J. Heterocycl. Chem., 1989, 26, 1167-1204.
- G.S. Lingaraju, T.C. Vinayaka, K.S.H. Kumar, M.P. Sadashiva, K.S. Rangappa, An Easy Access to 4,5-Disubstituted Thiazoles via Base- Induced Click Reaction of Active Methylene Isocyanides with Methyl Dithiocarboxylates, Synthesis, 2012, 44, 1373-1379
- Y. Tominaga, H. Okuda, S. Kohra, H. Mazume, Reaction of Enaminones with Carbon Disulfide: Synthesis of Heterocycles Using Enamino Dithiocarboxylates, J. Heterocycl. Chem., 1991, 28, 1245-1255.
- Y. Tominaga, Y. Matsuoka, Y. Onlyama, Y.Uchimura, H. Komiya, M. Hirayama, S. Kohra, A. Hosomi, Polarized Ethylenes. IV* Synthesis of Polarized Ethylenes Using Thioamides and Methyl Dithiocarboxylates and Their Application to Syntheses of Pyrazoles, Pyrimidines, Pyrazolo[3,4-d]pyrimidines and 5-aza[2.2.3]cyclazines, J. Heterocycl. Chem., 1990, 27, 647-660.
- O.M. Singh, Dithiocarboxylates and Related Compounds in the Synthesis of Heterocycles, Book: Targets in Heterocyclic Systems. Edition: Jointly Published with Societa Chimica Italiana, Italy, 2012, 265-308.
- E. S. H. El Ashry, A. A. Aly, M. R. Amer, M.R. Shah, S. W. Ng, Synthesis and X-Ray Analysis of Butyl and Glycosyl (2-aryl-amino-4,4-dimethyl-6-oxocyclohex-1-ene)carbodithioates and Their Possible Cyclization to 2-Thioxo-6,7-dihydro-1H-benzo[d][1,3]thiazine-5(2H)- one Derivatives, Carbohydr. Res., 2011, 346, 169-176.
- S-G. Zhang, C-G. Liang, W-H. Zhang, Recent Advances in Indazole-Containing Derivatives: Synthesis and Biological Perspectives, Molecules, 2018, 23, 2783-2823.
- L.J. Scott, Niraparib: First global approval, Drugs, 2017, 77, 1029-1034.
- R.S. baddman, N.U. Kumar, A.P. Reddy, R. Bandichhor, Regioselective methylation of indazole using methyl 2,2,2-trichloromethylacetimidate, Tetrahedron, 2013, 54, 1661- 1663.
- A.S. Al-Bogami, Mechanochemical synthesis of cyclohexanones and indazoles as potential agents, Res. Chem. Intermed., 2016, 42, 5457-5477.
- M. Gopalakrishnan, J. Thanusu, V. Kanagrajan, Design, synthesis, characterization and in vitro antimicrobial evaluation of 4,6-diaryl-4,5-dihydro-2-phenyl-2H-indazol-3-ols, J. Enzy. Inhib. Med. Chem., 2009, 24, 480-486.
- J.P- Villanueva, L. Y- Mulia, I. G- Sanchez, J. F.P-Espinosa, O.s- Artech, T. del R. S-Espunes, M.A. Cerbon, K.R-Villar, A.K.R. Vicente, M.C. Gines, Z.C-Galvan, D.B. E. Castro, Synthesis and biological evaluation of 2H- indazole derivatives: towards antimicrobial and anti-inflammatory dual agents, Molecules, 2017, 22, 1864-1878.
- Y. Wang, M. Yan, R. Ma, S. Ma, Synthesis and antibacterial activity of novel 4-bromo-1H- indazole derivatives as FtsZ inhibitors, Arch. Pharm. Chem. Life Sci., 2015, 348, 266-274.
- A. Chabukswar, B. Kuchekar, P. Lokhande, M. Tryambake, B. Pagare, V. Kadam, S. Jagdale, V. Chabukswar, Design, synthesis and evaluation of antibacterial activity of novel indazole derivatives, Curr. Bioactive Compd. 2013, 9, 263-269.
- N.M.Y. Elsayed, D. A.Abou El Ella, R. A. T. Serya, M. F. Tolba, R. Shalaby, K. A.M. Abouzid, Design, synthesis and evaluation of indazole- pyrimidine-based derivatives as anticancer agents with anti-angiogenic and antiproliferative activities, Med. Chem. Comm., 2016, 7, 881-899.
- N.M.Y. Elsayed, R. A. T. Serya, M. F. Tolba, M. Ahmed, K. Barakat, D. A. Abou El Ella, K. A.M. Abouzid, Design, synthesis, biological evaluation and dynamics simulation of indazole derivatives with antiangiogenic and antiproliferative anticancer activity, Bioorg.Chem., 2018, 82, 340-359.
- R. K. Bhatia, P. B. Raju, U. K. Jain, P. P. Kumar, K. Satyavathi, Synthesis and anti-inflammatory activities of 7-benzylidene-2,3-diphenyl-4,5,6,7-tetrahydro-2H-indazole derivatives, Res. J. Pharm. Biol. Chem. Sci., 2011, 2, 118-129.
- J. Liu, X. Peng, Y. Dai, W. Zhang, S.Ren, J. Ai, M. Geng, Y. Li, Design, synthesis, and biological evaluation of novel inhibitors bearing an indazole scaffold, Org. Biomol. Chem., 2015, 13, 7643- 7654.
- M. Yoo, M. Yoo, J. E. Kim, H. K. Lee, C. O. Lee, C. H. Park, K-Y. Jung, synthesis, and biological evaluation of indazole-4,7-dione derivatives as novel BRD4 inhibitors, Arch. Pharm. Res., 2018, 41, 46-56.
- E. Polo, J. Trilleras, J. Ramos, A. Galdamez, J. Quiroga, M. Gutierrez, Efficient MW-assisted synthesis, spectroscopic characterization, X-ray and antioxidant properties of indazole derivatives, Molecules, 2016, 21, 903-915.
- E.S.H. El Ashry, L.F. Awad, M. Nabil, M. O. Kh, Bdeewy, A Novel Trans Amination Process in 3- Arylamino-5,5-dimethylcyclohex-2-en-1-one with Nucleophiles and Antimicrobial Activities of Selected Products, Mediterr J. Chem., 2019, 7(6), 452-462.
- I. H. El Azab, L.M. Break, Z. A. A. El-Zahrani, Synthesis-based heterocyclic compounds and study their biological activity, Orient. J. Chem., 2016, 32, 2435- 2449.
- S. M. Ryadh, Enaminones as building blocks for the synthesis of substituted Pyrazoles with antitumor and antimicrobial activities, Molecules, 2011, 16, 1834-1853.
- A-Z. A. Elassar, A. A. El Khair, Recent developments in the chemistry of Enaminones, Tetrahedron, 2003, 59, 8463- 8480.
- E. S. H. El Ashry, M. R. Amer, A. A. Aly, M. Omer, O. M. Abdalla, A. A. Aly, S. Soomro, A. Jabeen, S.A. Halim, M. A. Mesaik, Z. Ul-Haq, Synthesis, biological evaluation and molecular docking studies of and benzyl, alkyl, glycosyl [2--(arylamino)-4,4-dimethyl-6-oxo-cyclohex-1- potential immunomodulatory and immune-suppressive agents, Bioorg. Med. Chem., 2012, 20, 3000- 3008.
- I. Jirkovsky, Studies on Enaminoketones, Can. J. Chem., 1974, 52, 55-65.
- J. V. Greenhill, J. Hanaee, P.J. Steel, Some reactions of Enaminones with
Isothiocyanates, J.Chem. Soc. Perkin Trans.1, 1990, 1869-1873.
DOI: http://dx.doi.org/10.13171/mjc761901713eshea
Refbacks
- There are currently no refbacks.
Copyright (c) 2019 Mediterranean Journal of Chemistry
