Optimization of textile azo dye degradation by electrochemical oxidation using Box-Behnken Design
Abstract
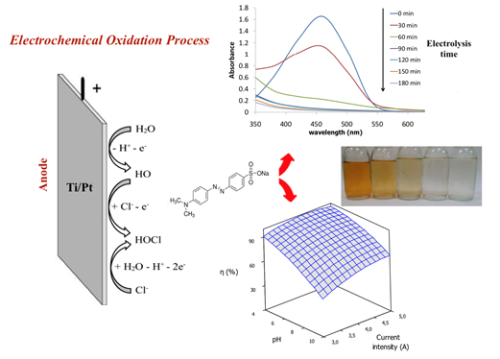
Degradation of textile azo dye solutions containing Methyl Orange by anodic oxidation using an electrochemical reactor was studied. The combined effect of independent parameters (current intensity, initial pH and electrolysis time) on color removal efficiency was investigated and optimized using response surface methodology. A Box-Behnken design was successfully employed for experimental design. The obtained quadratic model was statistically tested using analysis of variance (ANOVA). Results showed that the optimal operating conditions to achieve 98.51% efficiency for color removal were current intensity = 4.6 A, initial pH= 4 and electrolysis time = 65 min, at a dye concentration and temperature of 50 mg/L and 25 °C, respectively.
Full Text:
PDFReferences
- X. Li, X. Li, W. Yang, X. Chen, W. Li, B. Luo, K. Wang, Preparation of 3D PbO2 nanospheres@ SnO2 nanowires/Ti electrode and its application in Methyl Orange degradation, Electrochimica Acta, 2014, 146, 15-22.
- A. El-Ghenymy, F. Centellas, J. A. Garrido,
R. M. Rodreguez, I. Sires, P. L. Cabot, E. Brillas, Decolorization and mineralization of Orange G azo dye solutions by anodic oxidation with a boron-doped diamond anode in divided and undivided tank reactors, Electrochimica Acta, 2014, 130, 568-576.
- C. RamÃrez, A. Saldaña, B. Hernández,
R. Acero, R. Guerra, S. Garcia-Segura,
E. Brillas, J. M. Peralta-Hernández, Electrochemical oxidation of methyl orange azo dye at pilot flow plant using BDD technology, J. Ind. Eng. Chem., 2013, 19, 571-579.
- S. Raghu, C. W. Lee, S. Chellammal,
S. Palanichamy, C. A. Basha, Evaluation of electrochemical oxidation techniques for degradation of dye effluents-A comparative
approach, J. Hazard. Mater., 2009, 171,
-754.
- V. M. Vasconcelos, C. Ponce-de-León, J. L. Nava, M. R. V. Lanza, Electrochemical degradation of RB-5 dye by anodic oxidation, electro-Fenton and by combining anodic oxidation-electro-Fenton in a filter-press flow cell, Journal of Electroanalytical Chemistry, 2015, 765, 179-187.
- E. Sharifpour, E. Alipanahpour Dil, A. Asfaram, M. Ghaedi, A. Goudarzi, Optimizing adsorptive removal of malachite green and methyl orange dyes from simulated wastewater by Mnâ€doped CuOâ€Nanoparticles loaded on activated carbon using CCD-RSM: Mechanism, regeneration, isotherm, kinetic, and thermodynamic studies, Appl. Organomet. Chem., 2019, 33, e4768.
- N. Rahman, N. C. Dafader, A. R. Miah,
S. Shahnaz, Efficient removal of methyl orange from aqueous solution using amidoxime adsorbent, Int. J. Environ. Stud., 2018, 00,
-14.
- T. K. F. S. Freitas, C. A. Almeida, D. D. Manholer, H. C. L. Geraldino, M. T. F de Souza, J. C. Garcia, In Detox Fashion: waste water treatment; ed. by S.S. Muthu; Springer: Singapore, 2018, pp. 27-79.
- K. El Hassani, D. Kalnina, M. Turks, B. H. Beakou, A. Anouar, Enhanced degradation of an azo dye by catalytic ozonation over Ni-containing layered double hydroxide nanocatalyst, Sep. Sci. Technol., 2019, 210, 764-774.
- J. J. Murcia, Ã. C. Cely, H. A. Rojas, M. C. Hidalgo, J. A. NavÃo, Fluorinated and Platinized Titania as Effective Materials in the Photocatalytic Treatment of Dyestuffs and Stained Wastewater Coming from Handicrafts Factories. Catalysts., 2019, 9, 179.
- I. Sir, P. Llu, F. Centellas, A. Garrido, R. Mar, C. Arias, E. Brillas, Electrochemical degradation of clofibric acid in water by anodic oxidation Comparative study with platinum and boron-doped diamond electrodes, Electrochimica Acta, 2006, 52, 75-85.
- S.B. Dimitrijević, S.P. Dimitrijević, M.D. Vuković, Modern water treatment by electrochemical oxidation a review, Tmt, 2013, 10-11.
- K. Rajkumar, M. Muthukumar, Response surface optimization of electro-oxidation process for the treatment of CI Reactive Yellow 186 dye: reaction pathways, Appl. Water Sci., 2017, 7, 637-652.
- S. Cotillas, J. Llanos, P. Caćizares, D. Clematis, G. Cerisola, M. A. Rodrigo, M. Panizza, Removal of Procion Red MX-5B dye from wastewater by conductive-diamond electrochemical oxidation. Electrochimica Acta, 2018, 263, 1-7.
- I. Elaissaoui, H. Akrout, S. Grassini,
D. Fulginiti, L. Bousselmi, Effect of coating method on the structure and properties of a novel PbO2 anode for electrochemical oxidation of Amaranth dye. Chemosphere, 217, 2019, 26-34.
- E. Isarain-Chávez, M. D. Baró, E. Rossinyol, U. Morales-Ortiz, J. Sort, E. Brillas, E. Pellicer, Comparative electrochemical oxidation of methyl orange azo dye using Ti/Ir-Pb, Ti/Ir-Sn, Ti/Ru-Pb, Ti/Pt-Pd and Ti/RuO2 anodes, Electrochimica Acta, 2017, 244,
-208.
- F. Ghanbari, M. Moradi, In Advanced Nanomaterials for Wastewater Remediation; Ed by R.K. Gautam, M.C. Chattopadhyaya; CRC Press LLC: London, 2016.
- Faja.seppur.2017.01.A. S. Fajardo, R. C. Martins, D. R. Silva, R. M. Quinta-Ferreira, C. A. MartÃnez-Huitle, Electrochemical abatement of amaranth dye solutions using individual or an assembling of flow cells with Ti/Pt and Ti/Pt-SnSb anodes. Sep. Purif. Technol., 2017, 179, 194-203.
- M. Amini, H. Younesi, N. Bahramifar,
A. Akbar, Z. Lorestani, F. Ghorbani, A. Daneshi, M. Sharifzadeh, Application of response surface methodology for optimization of lead biosorption in an aqueous solution by Aspergillus niger, J. Hazard. Mater., 2008, 154, 694-702.
- R. O. Cristóvão, C. Gonçalves, C. M. Botelho, R. J. Martins, R. A. Boaventura, Chemical oxidation of fish canning wastewater by Fenton's reagent, J. Environ. Chem. Eng., 2014, 2, 2372-2376.
- R. H. Myers, D. C. Montgomery, C. M. Anderson-Cook, Response Surface Methodology: Process and Product Optimization using Designed Experiments; ed. by R. H. Myers; John Wiley & Sons: New Jersey, 2009.
- M. Danish, W. A. Khanday, R. Hashim, N. S. B. Sulaiman, M. N. Akhtar, M. Nizami, Application of optimized large surface area date stone (Phoenix dactylifera) activated carbon for rhodamin B removal from aqueous solution: Box-Behnken design approach, Ecotoxicol. Environ. Saf., 2017, 139, 280-290.
- L. Adlnasab, N. Shekari, A. Maghsodi, Optimization of arsenic removal with Fe3O4@ Al2O3@ Zn-Fe LDH as a new magnetic nano adsorbent using Box-Behnken design, J. Environ. Chem. Eng., 2019, 7, 102974.
- N. C. Fernandes, L. B. Brito, G. G. Costa, S. F. Taveira, M. S. S. Cunha-Filho, G. A. R. Oliveira, R. N. Marreto, Removal of azo dye using Fenton and Fenton-like processes: Evaluation of process factors by Box-Behnken design and ecotoxicity tests, Chem.-Biol. Interact., 2018, 291, 47-54.
- A. Alaoui, K. EL Kacemi, K. EL ass, A. Kitane. Application of Box-Behnken design to determine the optimal conditions of reductive leaching of MnO2 from manganese mine tailings, Trans. Indian Inst. Met., 2015, 56,
-141.
- H. Zhang, X. Ran, X. Wu, D. Zhang, Evaluation of electro-oxidation of biologically treated landfill leachate using response surface methodology, J. Hazard. Mater., 2011, 188, 261-268.
- H. Zhang, Y. Li, X. Wu, Statistical Experiment Design Approach for the Treatment of Landfill Leachate by Photoelectro-Fenton Process, Journal of Environmental Engineering, 2012, 138, 278-286.
- M. Ahmadi, F. Ghanbari, S. Madihi-Bidgoli, Photoperoxi-coagulation using activated carbon fiber cathode as an efficient method for benzotriazole removal from aqueous solutions: modeling, optimization and mechanism, J. Photochem. Photobiol. A. Chem., 2016, 322, 85-94.
- M.Moradi, F. Ghanbari, M. Manshouri, K. A. Angali, Photocatalytic degradation of azo dye using nano-ZrO 2/UV/Persulfate: Response surface modeling and optimization. Korean J. Chem. Eng., 2016, 33, 539–546.
- M. Moradi, F. Ghanbari, E.M. Tabrizi, Removal of Acid Yellow 36 using Box-Behnken designed photoelectro-Fenton : A study on removal mechanisms, Toxicol. Environ. Chem., 2015, 97, 700-709.
- H. Zhang, X. Ran, X. Wu, D. Zhang, Evaluation of electro-oxidation of biologically treated landfill leachate using response surface methodology, J. Hazard. Mater., 2011, 188, 261-268.
- F. Zhang, C. Feng, W. Li, J. Cui, Indirect electrochemical oxidation of dye wastewater containing Acid Orange 7 using Ti/RuO2-Pt electrode, Int. J. Electrochem. Sci., 2014, 9, 943-954.
- C. A. Martinez-Huitle, M. A. Rodrigo, I. Sires, O. Scialdone, Single and Coupled Electrochemical Processes and Reactors for the Abatement of Organic Water Pollutants: A Critical Review, Chem. Rev., 2015, 115, 13362-13407.
- D. A. Coledam, J. M. Aquino, R. C. Rocha-Filho, N. Bocchi, S. R. Biaggio, Influence of chloride-mediated oxidation on the electrochemical degradation of the direct black 22 dye using boron-doped diamond and β-PbO2 anodes, QuÃmica Nova, 2014, 37, 1312-1317.
- A. D. Hiwarkar, S. Singh, V. C. Srivastava,
I. D. Mall, Mineralization of pyrrole, a recalcitrant heterocyclic compound, by electrochemical method: Multi-response optimization and degradation mechanism, J. Environ. Manage., 2017, 198, 144-152.
- L. Labiadh, A. Barbucci, M. P. Carpanese,
A. Gadri, S. Ammar, M. Panizza, Direct and indirect electrochemical oxidation of Indigo Carmine using PbO2 and TiRuSnO2, J. Solid. State. Electrochem., 2017, 21, 2167-2175.
DOI: http://dx.doi.org/10.13171/mjc851907103ko
Refbacks
- There are currently no refbacks.
Copyright (c) 2019 Mediterranean Journal of Chemistry
