Quantitative structure-activity relationship (QSAR) investigation on 2-arylideneaminobenzimidazole derivatives as anti-proliferative activity against mv4-11 human leukaemia cells
Abstract
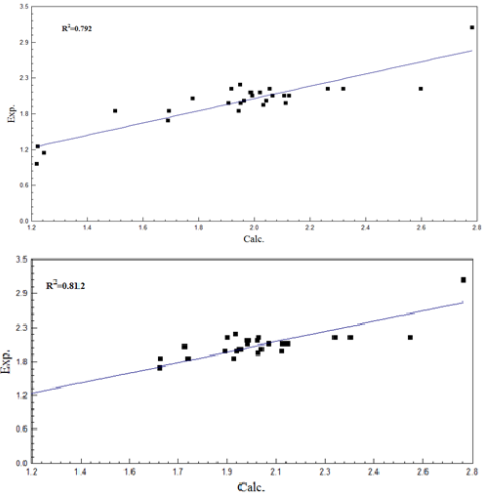
This undertaking involves QSAR investigations on the use of 28 2-arylideneaminobenzimidazole derivatives, for curbing the proliferative activity of cells in the mv4-11 human leukaemia cell line. The geometries of the compounds under investigation were initially optimized at level (PM3) in accordance to the semi-empirical theory, and subsequently through the B3LYP procedure at the 6-31G(d) basis set in accordance to the DFT theory. The multiple regression procedure was employed for the construction of two QSAR equations, to assess the anti-proliferative activity of these compounds (Equations 1 and 2). The values of R2 extended from 0.792 to 0.812, those of S from 0.187 to 0.193, and those of F from 24.897 to 30.429. According to the results attained, the use of four parameters in Equation 2[(N5), (N10), r(C1=N2) andLUMO+4] led to raised R2 values and minimized S values. This suggests that these parameters are significant for identifying the anti-proliferative effectiveness of the compounds, in the context of mv4-11 human leukaemia cell line cells. This revelation is also an indication that QSAR can be successfully applied for a broad range of compounds.Â
Full Text:
PDFReferences
- G. Aljohani, M.A. Said, D. Lentz, N. Basar, A. Albar, S.Y. Alraqa and Adeeb A. Ali, Microwave-Assisted Synthesis of Mono- and Disubstituted 4-Hydroxyacetophenone Derivatives via Mannich Reaction: Synthesis, XRD and HS-Analysis, Molecules, 2019, 24, 1-14.
- A. Balakrishnan and A. Sankar, Study on the Synthesis, Characterization and Antimicrobial Activity of the New Mannich Base Benzimidazolyl Phenyl Methyl Acetamide and its Metal Complexes, International Journal of Pharmacy and Pharmaceutical Research, 2017, 10(2), 60-68.
- G. Jinky, C. Dipak, K.K. Mukesh, R. Mithun, Synthesis and Antimalarial Activity Evaluation of some Mannich Bases of Tetraoxane-Phenol Conjugate, Indian Journal of Pharmaceutical Education and Research, 2016, 50(4), 591-597.
- A.K. Keshari, A. Tewari, S.S. Verma, S.K. Saraf, Novel Mannich-bases as Potential Anticonvulsants: Syntheses, Characterization and Biological Evaluation, Central Nervous System Agents in Medicinal Chemistry, 2017, 17(3), 219-228.
- K. Kamioski, J. Obniska, I. Chlebek, P. Liana, E. PIkala, Synthesis and biological properties of new N-Mannich bases derived from 3-methyl-3-phenyl- and 3,3-dimethyl-succinimides. Part V, Eur. J. Med. Chem., 2013, 66,12-21.
- S. Linz, J. Muller, H. Hubner, P. Gmeiner, R. Troschutz, Design, synthesis and dopamine D4 receptor binding activities of new N-heteroaromatic 5/6-ring Mannich bases, Bioorg. Med. Chem., 2009, 17, 4448-4458.
- T. Plech, M. Wujec, M. Majewska,
U. Kosikowska, A. Malm, microbiologically active Mannich bases derived from 1,2,4-triazoles. The effect of C-5 substituent on antibacterial activity, Med. Chem. Res., 2013, 22, 2531-2537.
- A. Idhayadhulla, R.S. Kumar, A.J. Abdul Nasser, J. Selvin and A. Manilal, Synthesis of some Mannich base derivatives and their antimicrobial activity study, Arabian Journal of Chemistry, 2014, 7, 994-999.
- S.N. Kanchana, V. Burra and L.K. Ravindra Nath, Novel Synthesis and Anti-Microbial Activity Study of Innovative Mannich Bases Containing 2-Phenoxy-1,3, 2-dioxa Phospholanes and Indole Systems, 2014, 30(3), 1349-1360.
- L. Popiołek, A. Biernasiuk, K. Paruch, P. Patrejko, M. Wujec, Synthesis and evaluation of antimicrobial properties of new Mannich bases of 4,5-disubstituted-1,2,4-triazole-3-thiones,2014, 192(7), 880-885.
- V. Ravichandran, R. Harish, QSAR studies on imidazoles and sulfonamides as antidiabetic agents, Ovidius University Annals of Chemistry, 2019, 30(1), 5-13.
- M.T. Ibrahim, A. Uzairu, G.A. Shallangwa, S. Uba, QSAR modelling and docking analysis of some thiazole analogues as âº-glucosidase inhibitors, The Journal of Engineering and Exact Sciences, 2019, 5(3), 257-270.
- O.A. Hatem, Computational Chemistry Application of Physicochemical Descriptors: QSAR Study on Some β-Carboline Compounds, Der Pharma Chemica, 2017, 9(8), 150-156.
- N.A. Saleh, The QSAR and docking calculations of fullerene derivatives as HIV-1 protease inhibitors, Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 2015, 136, 1523-1529.
- J.H. Al-Fahemi, D.L. Cooper, N.L. Allan, CroaticaChemicaActa, Predictions of Toxicity to Chlorella vulgaris and the Use of Momentum-space Descriptors, 2009, 82, 311-316.
- L. Chang, C.M. Chang, A QSAR Study on the Persistence of Fungicides in the Environment, International Journal of Quantitative Structure-Property Relationships, 2019, 4(2),100-116.
- W. Shao-peng, J. Zhi-qin, Z. Hui-xiao, Z. Ji-wen, W. Yong-Hua, W. Wen-jun, Isolation, biological evaluation and 3D-QSAR studies of insecticidal/narcotic sesquiterpene polyol esters, Journal of Molecular Modeling, 2011, 17, 681-693.
- K.A. Hussain, W. A-H Radhi, S. M-H. Ismael, Quantitative Structure-Activity Relationships (QSAR) study and improving it of some Schiff-base ligands as anticancer for prostate cancer, Journal of Chemical and Pharmaceutical Research, 2012, 4, 1702-1707.
- B. Bultink, W. Langenaeker, R. Carbo-dorca, J.P. Tollenaere, Fast Calculation of Quantum Chemical Molecular Descriptors from the Electronegativity Equalization Method. J. Chem. Inf. Compt. Sci., 2003, 43, 422-428.
- Y. Xia, D. Yin, C. Rong, Q. Xu, D. Yin, S. Liu, Impact of Lewis Acids on Diels−Alder Reaction Reactivity: A Conceptual Density Functional Theory Study, J. Phys. Chem. A, 2008, 112, 9970-9977.
- A.G. Alex, Firefly version 8, http://classic.chem.msu.su/gran/firefly/index.htm.
- A.D. Becke, Density-functional thermochemistry. V. Systematic optimization of exchange-correlation functionals, Density-functional thermochemistry. V. Systematic optimization of exchange-correlation functionals, J. Chem. Phys., 1997, 107, 8554-8560.
- C. Lee, W. Yang, R.G. Parr, Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density, Phys. Rev. B: Contents. Matter Mater. Phys., 1988, 37, 785-789.
- A. Nowicka, H. Liszkiewicz, W. Nawrocka, J. Ietrzyk, J. Sadowska, Synthesis and in vitro antiproliferative activity of novel 2- arylidene-amino benzimidazole, Acta Polonia pharmaceutical - drug research, 2015, 72, 951-963.
- K. Baumann, Uniform-length molecular descriptors for quantitative structure-property relationships (QSPR) and quantitative structure-activity relationships (QSAR): classification studies and similarity searching, J. Anal. Chem., 1999, 18, 36-41.
- R. Veerasamy, H. Rajak, A. Jain, S. Sivadasan, C. P. Varghese and R.K. Agrawal, Validation of QSAR Models - Strategies and Importance, International Journal of Drug Design and Discovery, 2011, 2(3), 511-519.
- D.L. Massart, B.G. Vandeginste, L.M. Buydens, S.D. Jong, P.J. Lewi, J. Smeyers-Verbeke, “Handbook of Chemometrics and Qualimetrics: Part Aâ€, Elsevier, Amsterdam,1997.
- W.A. Radhi, S.M.H. Ismael, J.M. Al-Shawi, K.A. Hussein, Quantitative Structure-Activity Relationship Studies of Flavonoids Substituted as Anticancer Agents Activity against the Growth of the Hepatic Cancer Cell lines HepG2, International Journal of Chemistry, 2017, 9, 1-9.
DOI: http://dx.doi.org/10.13171/mjc92190909835smhi
Refbacks
- There are currently no refbacks.
Copyright (c) 2019 Mediterranean Journal of Chemistry
